Classify the statements about redox reactions as true or false: an engaging exploration into the fundamental principles of redox chemistry. Join us as we delve into the fascinating world of oxidation and reduction, unraveling the intricacies of these reactions that shape our understanding of chemical processes.
Redox reactions, short for reduction-oxidation reactions, play a pivotal role in numerous chemical, biological, and industrial processes. They involve the transfer of electrons between atoms or ions, leading to changes in their oxidation states. Understanding redox reactions is crucial for comprehending a wide range of phenomena, from combustion and respiration to the functioning of batteries and fuel cells.
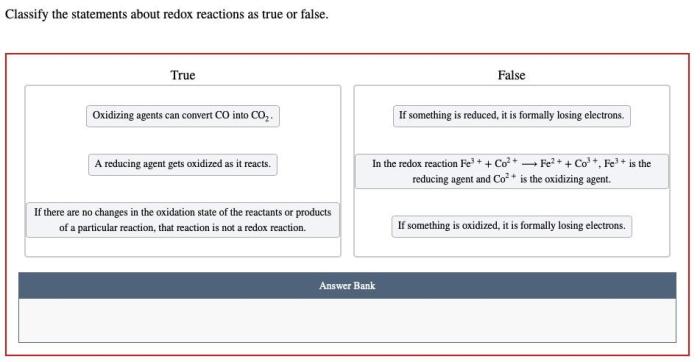
Redox Reactions Overview: Classify The Statements About Redox Reactions As True Or False
Redox reactions, short for reduction-oxidation reactions, are chemical reactions that involve the transfer of electrons between atoms or ions. These reactions play a crucial role in various fields, including chemistry, biology, and medicine.
Redox reactions can be understood by examining the concept of oxidation and reduction. Oxidation refers to the loss of electrons by an atom or ion, while reduction refers to the gain of electrons. The species that undergoes oxidation is known as the reducing agent, while the species that undergoes reduction is known as the oxidizing agent.
Classifying Redox Reactions
Redox reactions can be classified based on several criteria, including the type of species involved and the nature of the electron transfer. One common way to classify redox reactions is to distinguish between oxidation-reduction reactions and non-oxidation-reduction reactions.
Oxidation-reduction reactions involve the transfer of electrons between atoms or ions, resulting in a change in their oxidation states. In contrast, non-oxidation-reduction reactions do not involve electron transfer and do not result in changes in oxidation states.
Common types of redox reactions include:
- Combustion reactions: These reactions involve the reaction of a substance with oxygen, resulting in the formation of oxides.
- Redox reactions in aqueous solutions: These reactions involve the transfer of electrons between ions in water.
- Electrochemical reactions: These reactions involve the transfer of electrons between electrodes in an electrochemical cell.
True or False Statements
| Statement | True/False |
|---|---|
| Redox reactions always involve the transfer of electrons. | True |
| Oxidation is the loss of electrons. | True |
| The reducing agent is the species that undergoes oxidation. | False |
| Non-oxidation-reduction reactions do not involve electron transfer. | True |
| Combustion reactions are a type of redox reaction. | True |
Additional Examples
Additional examples of redox reactions include:
- The rusting of iron: Iron reacts with oxygen and water to form iron oxide (rust).
- The photosynthesis in plants: Plants use sunlight to convert carbon dioxide and water into glucose and oxygen.
- The digestion of food in the human body: Food is broken down into smaller molecules through a series of redox reactions.
Applications of Redox Reactions, Classify the statements about redox reactions as true or false
Redox reactions have numerous applications in various fields:
- Chemistry: Redox reactions are used in the production of metals, batteries, and fuels.
- Biology: Redox reactions are essential for cellular respiration, photosynthesis, and other metabolic processes.
- Medicine: Redox reactions are involved in drug metabolism, antioxidant defense, and the treatment of diseases.
FAQ Explained
What is the key difference between oxidation and reduction?
Oxidation involves the loss of electrons, while reduction involves the gain of electrons.
How can I identify a redox reaction?
Look for changes in oxidation states of atoms or ions, or the presence of oxidizing or reducing agents.
What are some common applications of redox reactions?
Redox reactions are used in batteries, fuel cells, combustion engines, and many industrial processes.


